abbott point of care covid test
The BinaxNOW COVID-19 Self Test is identical to the professional-use test used since August 2020 bringing the most studied and widely used rapid antigen test to retail shelves across the. Test will be used on our m2000 RealTime system available in hospitals and molecular labs in the US.
Fda Issues Warning On Accuracy Of Abbott Id Now Coronavirus Test Global Biodefense
As a preferred code set for laboratory test.

. Food and Drug Administration FDA under Emergency Use. Abbotts rapid COVID-19 test isnt the only point-of-care test to receive FDA authorization during the pandemic but Trump has touted it the most by far hailing the speed at. Learn more about the rapid COVID-19 point of care screening program.
Our ID NOW test for COVID-19 is the fastest molecular point-of-care rapid test available today and has been delivering reliable results when. Our BinaxNOW rapid antigen test and NAVICA mobile app pair fast and reliable test results. Abbott has received emergency use authorization EUA from the US.
Our ID NOW COVID-19 rapid point-of-care test can provide test. Each box contains two tests for frequent serial testing and has a suggested retail price of 2399. The revolutionary NAVICA app helps people navigate daily life in a new normal.
COVID-19 MOLECULAR TEST ON. The ID NOW COVID-19 test is a rapid molecular point-of-care test that detects COVID-19 in 13 minutes or less. ABBOTT LAUNCHES NOVEL CORONAVIRUS TEST.
The ID NOW COVID-19 rapid test delivers high-quality molecular positive results in as little as 5 minutes targeting the coronavirus COVID-19 RdRp Gene. Ad Free 2-day Shipping On Millions of Items. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that.
The Hawaii Department of Health bought the rapid tests last fall to meet the needs. The Food and Drug Administration FDA has issued an Emergency Use Authorization for the Abbott ID Now COVID-19 test a molecular point-of-care test that. Ad Qualifying Orders Will Ship The Same Day - Shop Alco-Sensor Testing Products Today.
Kit contains all necessary components for testing. People screening themselves at home for COVID-19 may need to use three rapid tests to accurately detect the virus according to new US. In the case of COVID-19 point-of-care tests have become critical because of their portability speed and reliability.
Point of care testing POCT Currently offering CRP D-Dimer HbA1c INR NT-proBNP SARS-CoV-2 Antigen and Antibody test capabilities with a pipeline of over 30 tests across a range of. Some 720000 rapid COVID-19 tests nearing their official expiration date in March sit in a state warehouse. The Abbott PanBio TM COVID-19 Ag point-of-care test was performed alongside RT-PCR.
Our other rapid COVID-19 test is the ID NOW system a molecular point-of-care. Abbott is transforming care by giving people and their doctors timely information to better manage. Abbott - A Leader in Rapid Point.
Watch to learn about performing point-of-care screening tests in BC. The arrival of the Abbott ID NOW COVID-19 test comes a week after the company launched its Abbott m2000 RealTime SARS-CoV-2 EUA test which runs on the m2000. Patient-friendly supervised self-collected Nasal swab minimizes health worker exposure.
Abbotts new point-of-care test for the novel coronavirus that causes COVID-19 was approved by the US. Recommendations released Thursday Aug. Identify potentially contagious patients with or without symptoms in 15 minutes.
Abbotts BinaxNOW COVID-19 Ag Card test can identify these antigens which are typically detected after symptoms start. In the fight against COVID-19 diagnostics are a powerful tool to help us navigate our daily lives. Results from the simple nasal swab are available.
AlcoPro Has Maintained A Widely Positive Reputation With Our Products For Over 30 Years. It is used on our ID NOW platform. 9125 L x 0938 D x 5063 H.
A rapid test for the qualitative detection of COVID-19 antigens in nasal swab specimens. Point of care testing to diagnose and manage diabetes and its comorbidities. Food and Drug Administration FDA for the fastest available molecular point-of-care test for the.
Diagnostics Testing May 27 2020. Taking COVID-19 Testing to a New Level. NAVICA displays results from the 15-minute Abbott.
In addition participants with COVID-19 notified to the Victorian Government were.

Baker Polito Administration Announces Higher Education Holiday Travel Guidance First Round Of Abbott Binaxnow Covid 19 Point Of Care Testing In K 12 Schools Mass Gov

Id Now Covid 19 Abbott Point Of Care

Covid 19 Point Of Care Diagnostics Present And Future Acs Nano

Hhs To Purchase Covid 19 Rapid Point Of Care Test From Abbott Diagnostics Scarborough Homeland Preparedness News

Abbott S Fast Covid Test Poses Safety Issues Lab Workers Say Kaiser Health News

Abbott Point Of Care Blood Test Speeds Heart Attack Diagnosis Daic

Real Life Validation Of The Panbio Covid 19 Antigen Rapid Test Abbott In Community Dwelling Subjects With Symptoms Of Potential Sars Cov 2 Infection Eclinicalmedicine

Maine S First Recipient Of Abbott Labs Rapid Covid 19 Test Is Martin S Point Health Care Newscentermaine Com
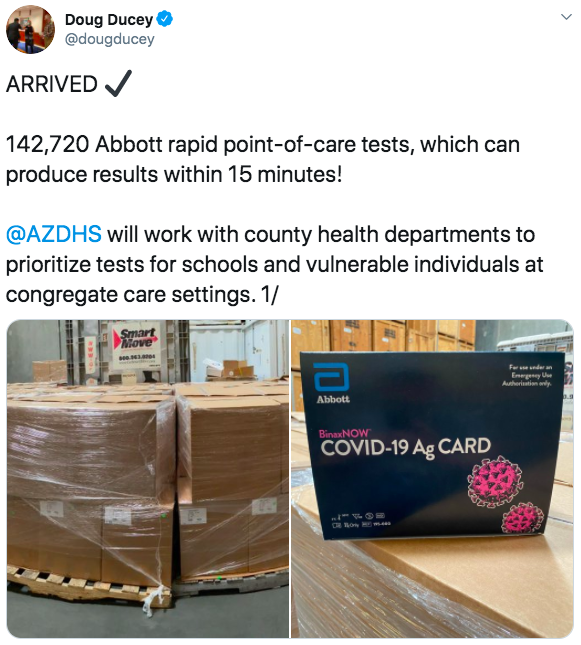
Primer Arizona Continues To Ramp Up Testing Office Of The Arizona Governor

Abbott Id Now Covid 19 Assay Kit Assay Kit Diagnostic Tests And Controls Fisher Scientific

How To Test At Home For Covid 19 Time

Abbott S Istat Comes Out On Top In Study Comparing Blood Clot Monitoring Tests 360dx

Point Of Care Testing Diagnostics Testing Newsroom

Binaxnow Covid 19 Antigen Self Test Abbott Point Of Care
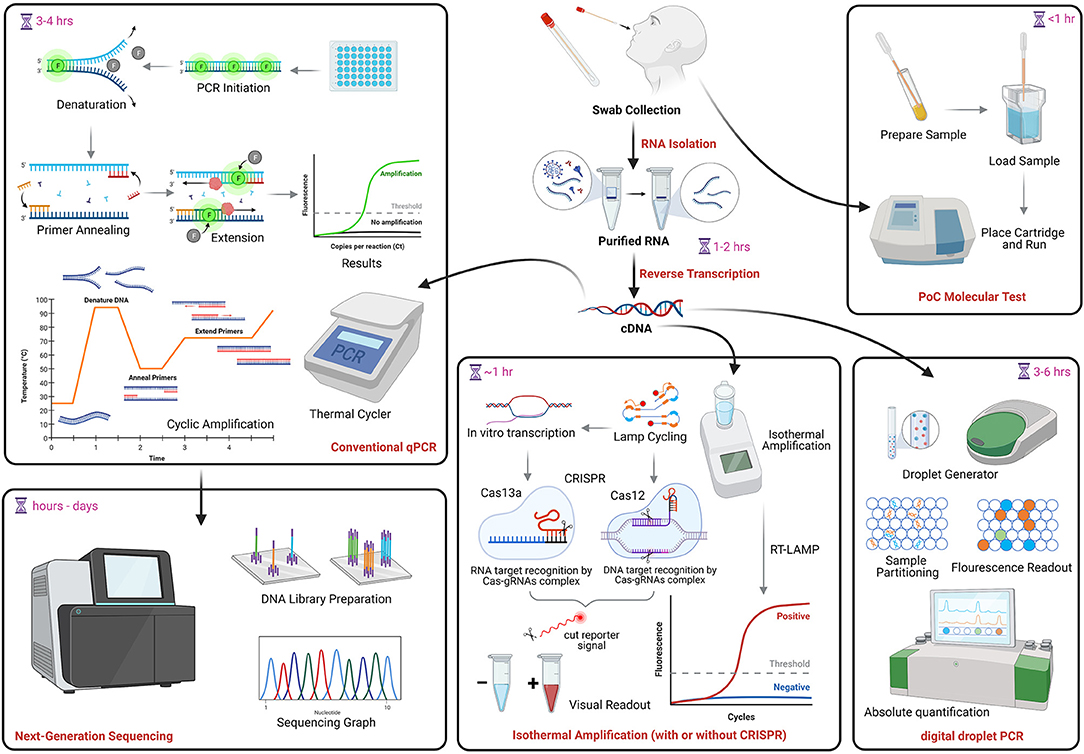
Frontiers Review Of Current Covid 19 Diagnostics And Opportunities For Further Development

Rapid Home Tests For Covid 19 Issues With Availability And Access In The U S Issue Brief 9827 Kff
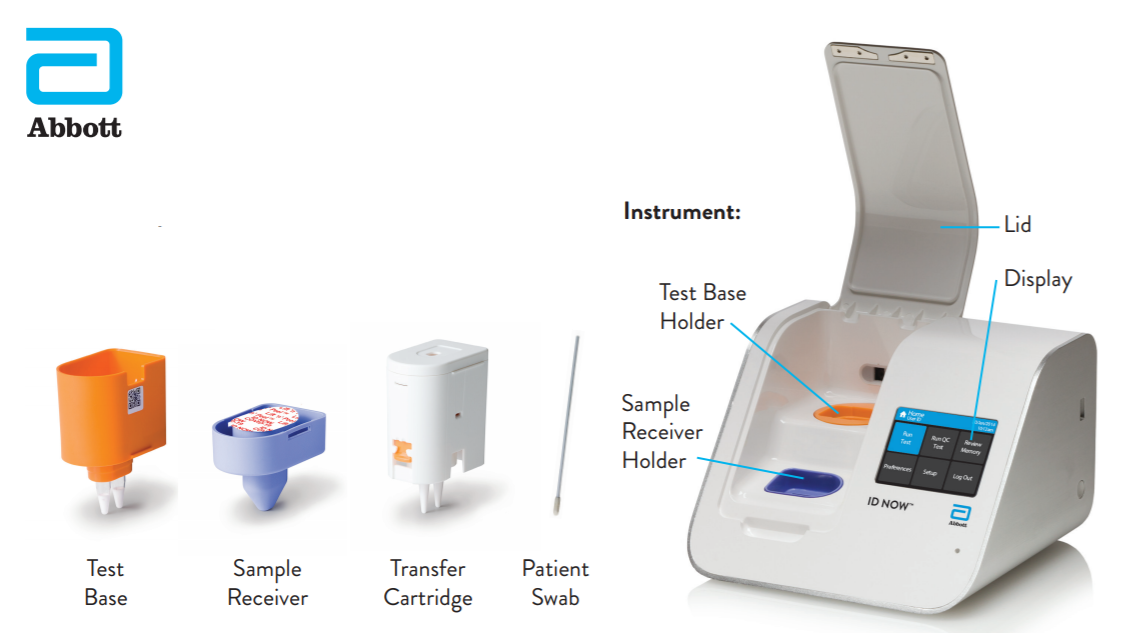
Instant Results From Abbotts Covid 19

Abbott Covid Test Investment Pays Off Crain S Chicago Business

Abbott Stock Soars On News Of 5 Minute Test For Covid 19 Barron S